Abstract
Introduction Thrombotic microangiopathies (TMA) include a range of rare and life-threatening disorders that present as thrombocytopenia, microangiopathic hemolytic anemia and end organ damage. Atypical hemolytic uremic syndrome (aHUS) is caused by dysregulation of the alternative complement pathway and is diagnosed by excluding thrombotic thrombocytopenia purpura (TTP), STEC-HUS and other TMAs. Whereas TTP is characterized by a severe deficiency (<10%) in ADAMTS13 activity, aHUS is invariably associated with ADAMTS13 >10%. The PLASMIC score (PS) was developed to rapidly predict the presence of severe ADAMTS13 deficiency using seven commonly available clinical variables (see figure legend). A PS of ≥6 (min-max 0-7) suggests a high probability of severe ADAMTS13 deficiency, and thus TTP. The objective of this study was to determine the utility of PS as part of the diagnostic pathway for aHUS. The rapid identification of patients with probable aHUS would facilitate the deployment of definitive therapy for these individuals and improve clinical outcomes.
Methods Patient data were obtained from the US Premier Healthcare Database, which contains de-identified patient-level health information from standard hospital discharge files, including patient demographics, diagnosis and laboratory information. Patients included were ≥18 years of age; had ≥1 inpatient Premier healthcare encounter between 01 January 2016 and 30 September 2020; a TMA-related (hemolytic anemia, thrombocytopenia or TMA) ICD-10 diagnosis code at ≥1 inpatient or hospital-based outpatient encounter; ≥1 valid and usable ADAMTS13 activity test result collected on Days 0-8 of the inpatient healthcare encounter; and valid and usable laboratory results for calculating PS. Key exclusion criteria included: patients only seen in the outpatient setting; plasmapheresis/plasma infusion and/or bilirubin >15 mg/dL prior to ADAMTS13 activity assay; and STEC-HUS diagnosis.
From these patient data, PSs were calculated (laboratory specimens collected on Days 0-3 of the index healthcare encounter). The primary analysis was conducted in the possible aHUS-RI cohort, which included patients with diagnosed TMA and renal impairment (RI; defined as serum creatinine > upper limit of normal within Days 0-8 of the healthcare encounter); a sensitivity analysis was conducted in all eligible patients after removing the RI requirement. Ground truth for aHUS was defined as patients who met the inclusion/exclusion criteria and had ADAMTS13 activity ≥10%. The diagnostic performance of the PS for identifying aHUS (PS ≤5) were summarized as sensitivity, specificity, positive and negative predictive values.
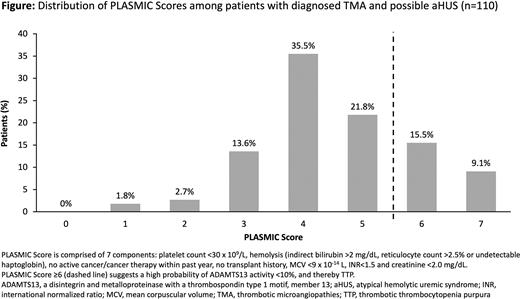
Results In the possible aHUS-RI patient cohort (n=110), most patients (n=83, 75.4%) scored between 0-5 on the PS scale with 70.9% (n=78) of these patients scoring 3-5; 24.6% (n=27) of patients had a PS of 6-7, suggestive of TTP diagnosis (Figure). In distinguishing aHUS-RI patients from those with other TMAs, a PS of ≤5 demonstrated a sensitivity of 86.5% (95% CI: 79.4%-93.6%); specificity of 71.4% (52.1%-90.8%); positive predictive value of 92.8% (87.2%-98.3%); and negative predictive value of 55.6% (36.8%-74.3%) in the primary analysis, which was supported by further sensitivity analyses.
Among PS components, platelet count and creatinine thresholds were met by the fewest patients in the possible aHUS-RI cohort (37.3% and 52.7%, respectively). Median ADAMTS13 activity level (Q1, Q3) was 64% (46, 84) in patients with PS of 0-5 and 5% (5, 55) in patients with PS 6-7.
Conclusion These real-world data show that PS ≤5 is observed in the majority (75.4%) of patients with possible aHUS-RI, which correlated with an average ADAMTS13 of ≥10%. Since tests for assessing ADAMTS13 activity are often not easily accessible, PS may be a useful tool to assist physicians in identifying patients with aHUS and enabling earlier treatment decisions.
Disclosures
Gasteyger:Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in private company. Uriol-Rivera:Alexion: Consultancy, Honoraria. Ernst:CTI Clinical Trial and Consulting Services: Current Employment. Booth III:CTI Clinical Trial and Consulting Services: Current Employment. Comas:Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in private company. Lum:Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in private company. Tomazos:Alexion, AstraZeneca Rare Disease: Current equity holder in private company, Ended employment in the past 24 months. Wang:Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in private company. Bendapudi:Alexion: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.